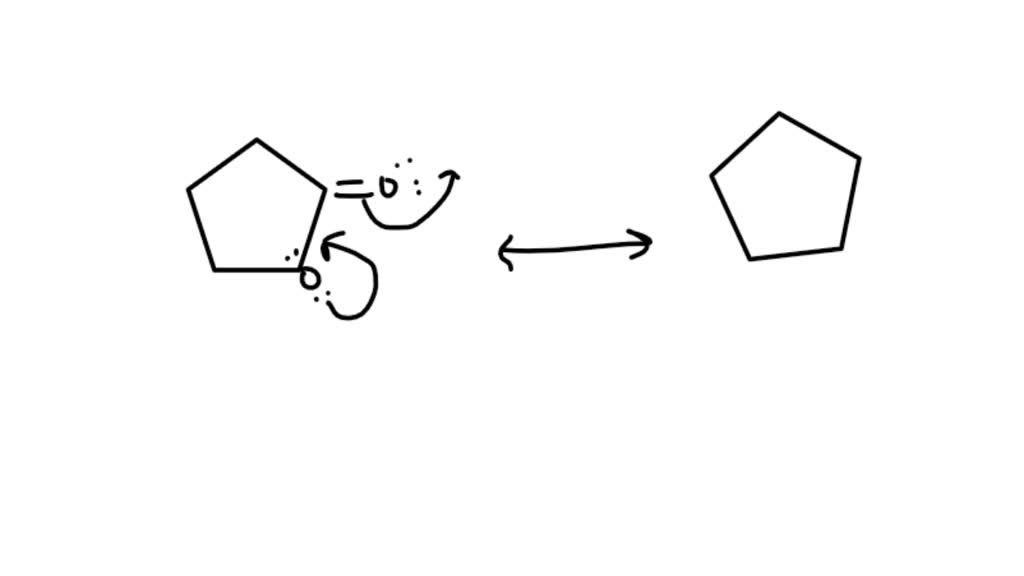
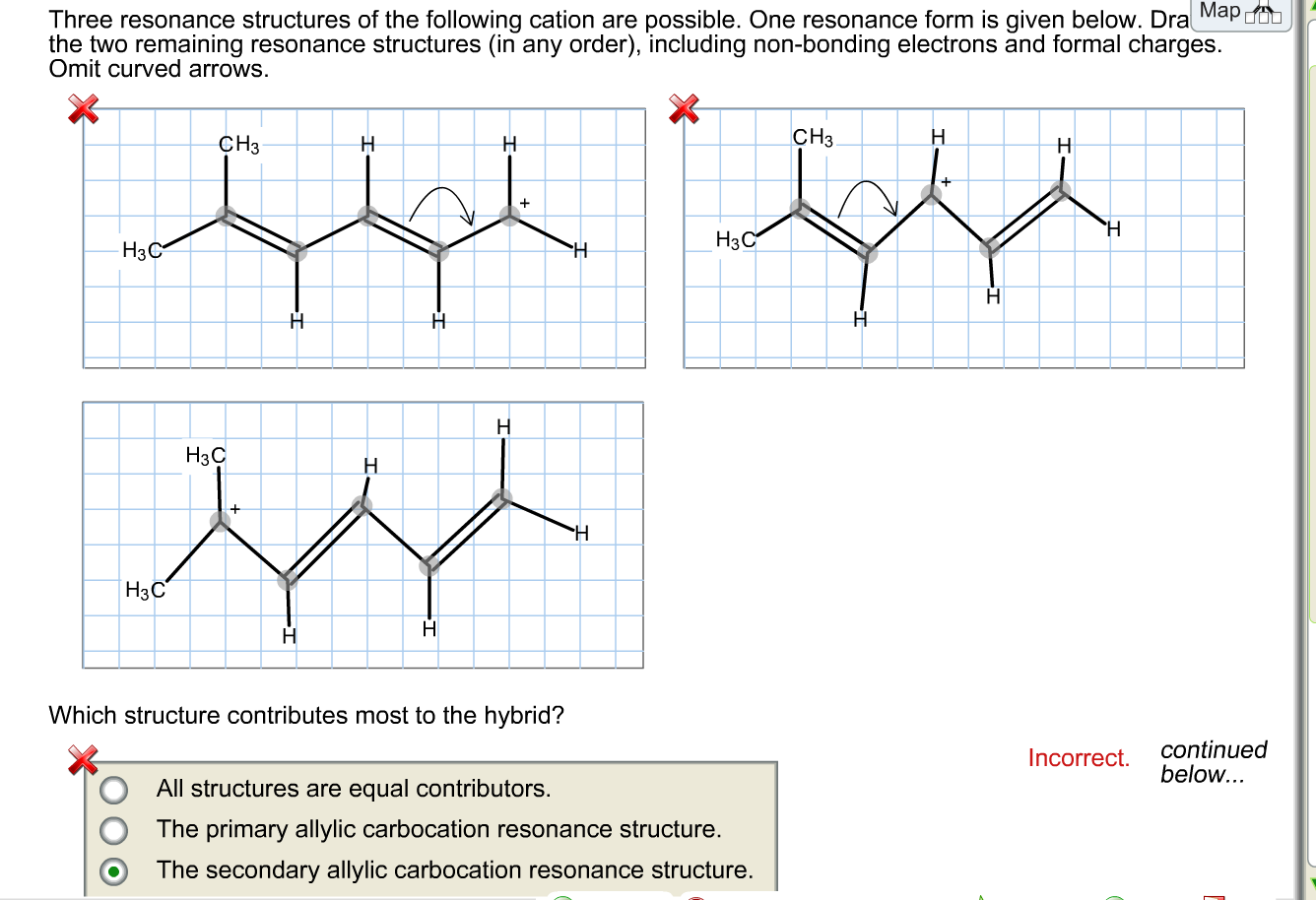
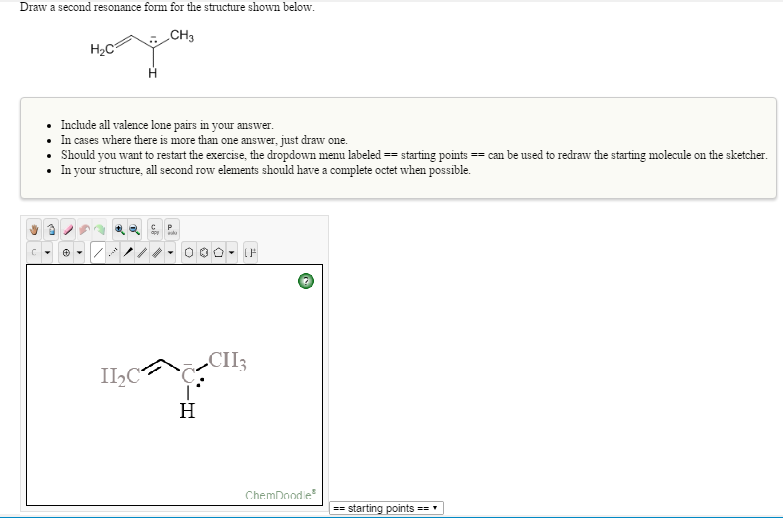
Draw A Second Resonance Form For The Structure Shown Below - A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. Each atom should have a complete valence shell and be shown with correct formal charges. There are 2 steps to solve this one. Draw a second resonance form for the structure shown below. • include all valence lone pairs in. The objective of this question is to draw the resonance forms for the given molecules.
Draw a second resonance form for the structure shown below. Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence. There are 2 steps to solve this one. The objective of this question is to draw the resonance forms for the given molecules. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. • include all valence lone pairs in.
Each atom should have a complete valence shell and be shown with correct formal charges. The objective of this question is to draw the resonance forms for the given molecules. There are 2 steps to solve this one. • include all valence lone pairs in. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. A carbocation (carbon with only 6 valence. Draw a second resonance form for the structure shown below.
SOLVED Draw second resonance form for the structure shown below
• include all valence lone pairs in. Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. Draw a second resonance form for the structure shown.
SOLVED Draw second resonance form for the structure shown below
Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. There are 2 steps to solve this one. The objective of this question is to draw the resonance forms for the given molecules. The resonance hybrid structure is drawn by the combination of two resonance.
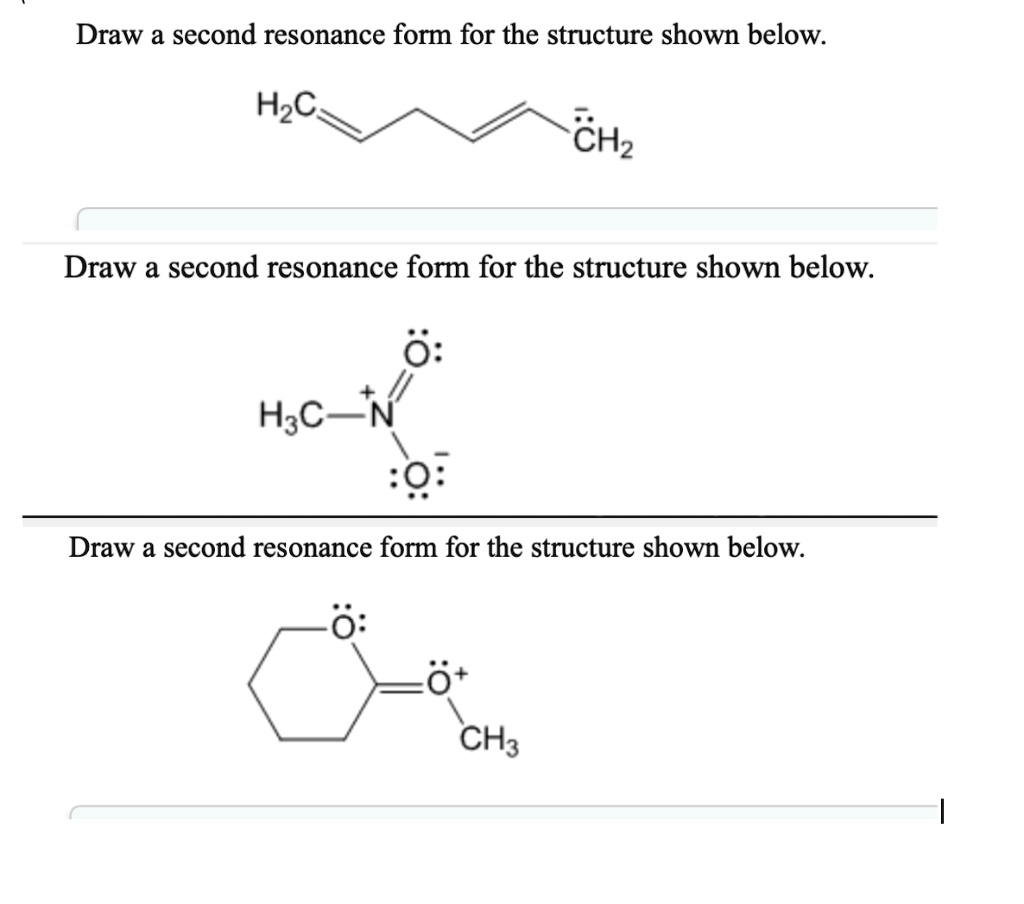
[Solved] Draw a second resonance form for the structure shown below. H
A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. The objective of this question is.
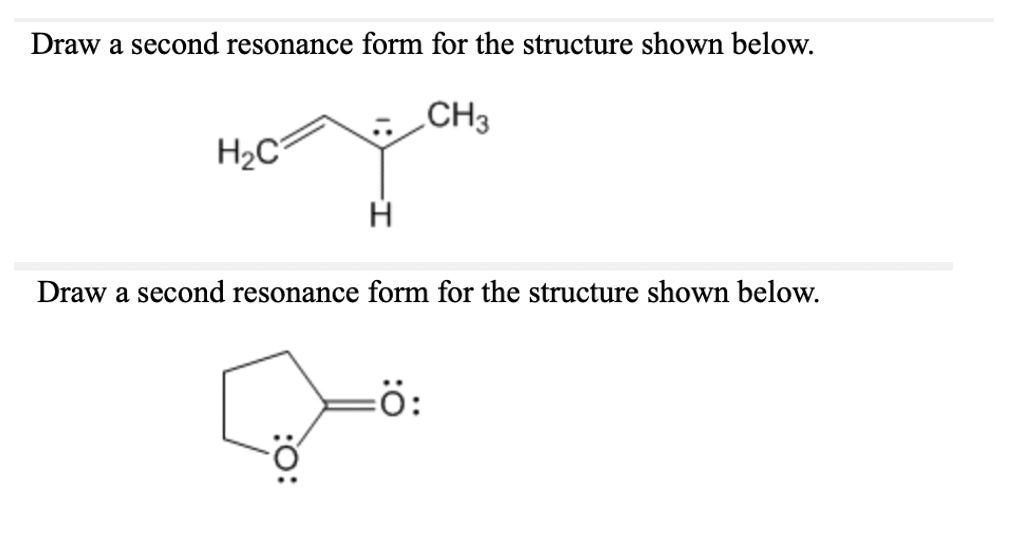
SOLVED Draw a second resonance form for the structure shown below CH3
There are 2 steps to solve this one. Draw a second resonance form for the structure shown below. • include all valence lone pairs in. Each atom should have a complete valence shell and be shown with correct formal charges. The objective of this question is to draw the resonance forms for the given molecules.
[Solved] Draw a second resonance form for the structure shown below. H
A carbocation (carbon with only 6 valence. Each atom should have a complete valence shell and be shown with correct formal charges. • include all valence lone pairs in. There are 2 steps to solve this one. Draw a second resonance form for the structure shown below.
Solved Draw a second resonance form for the structure shown
A carbocation (carbon with only 6 valence. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. There are 2 steps to solve this one. • include all valence lone pairs in. The objective of this question is to draw the resonance forms for the given molecules.
SOLVED Draw a second resonance form for the structure shown below H3C
A carbocation (carbon with only 6 valence. Each atom should have a complete valence shell and be shown with correct formal charges. The objective of this question is to draw the resonance forms for the given molecules. • include all valence lone pairs in. There are 2 steps to solve this one.
Draw A Second Resonance Form For The Structure Shown Below
There are 2 steps to solve this one. Draw a second resonance form for the structure shown below. The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. The objective of this question is to draw the resonance forms for the given molecules. • include all valence lone.
Solved Draw a second resonance form for the structure shown
A carbocation (carbon with only 6 valence. Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below. The objective of this question is to draw the resonance forms for the given molecules. There are 2 steps to solve this one.
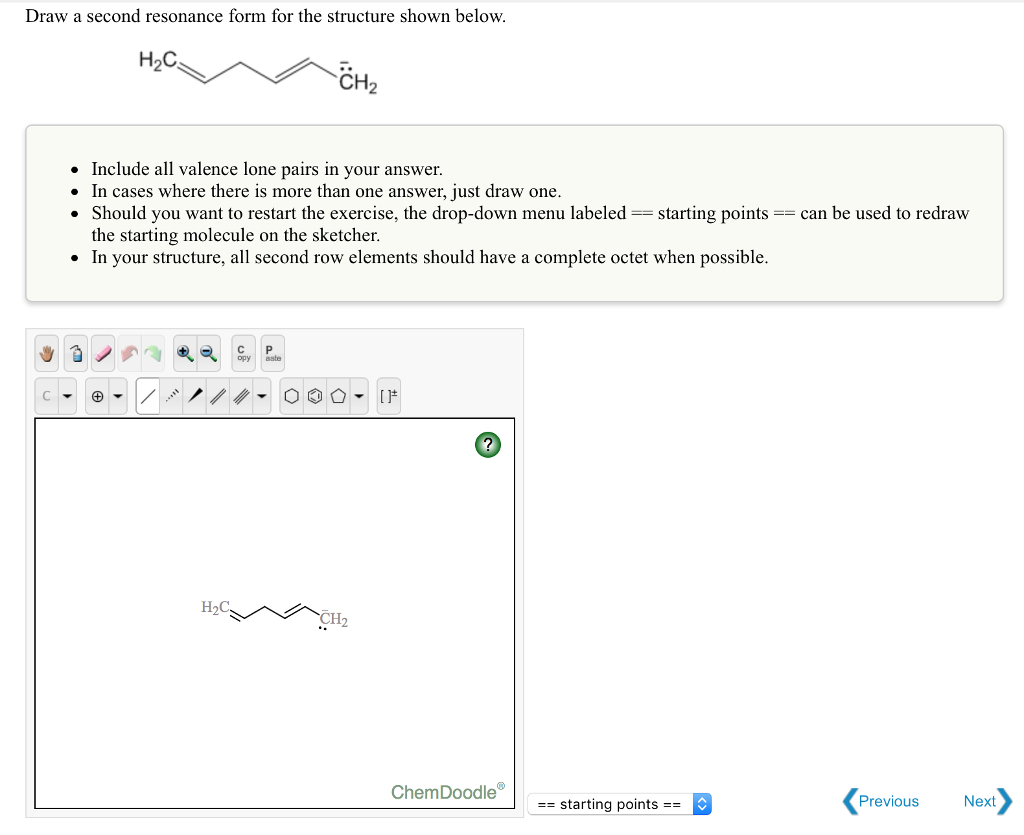
SOLVED Draw a second resonance form for the structure shown below HzC
The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen. A carbocation (carbon with only 6 valence. The objective of this question is to draw the resonance forms for the given molecules. Draw a second resonance form for the structure shown below. Each atom should have a complete.
• Include All Valence Lone Pairs In.
A carbocation (carbon with only 6 valence. The objective of this question is to draw the resonance forms for the given molecules. Each atom should have a complete valence shell and be shown with correct formal charges. Draw a second resonance form for the structure shown below.
There Are 2 Steps To Solve This One.
The resonance hybrid structure is drawn by the combination of two resonance structures, giving a partial negative charge on both the oxygen.